Research > Focus B > Work Packages B03
Summary
This project aims at evaluating and targeting immuno-relevant glioma cell-intrinsic and -extrinsic factors and microenvironmental immunomodulation to develop combinatorial targeted (immuno-) therapies for isocitrate dehydrogenase (IDH) mutant gliomas. The strategic aim is to utilize immuno-relevant IDH-associated as well as tertiary and quaternary genetic event-associated vulnerabilities in pathophysiologically optimized glioma models for rationalizing combinatorial clinical trials. The visual abstract below shows the aims and planned tasks of the work package.
Task 1
Multiomic characterization and targeting of the immune microenvironment specific for tertiary and quaternary genetic alterations in IDH-mutant gliomas
Steps / Workflow
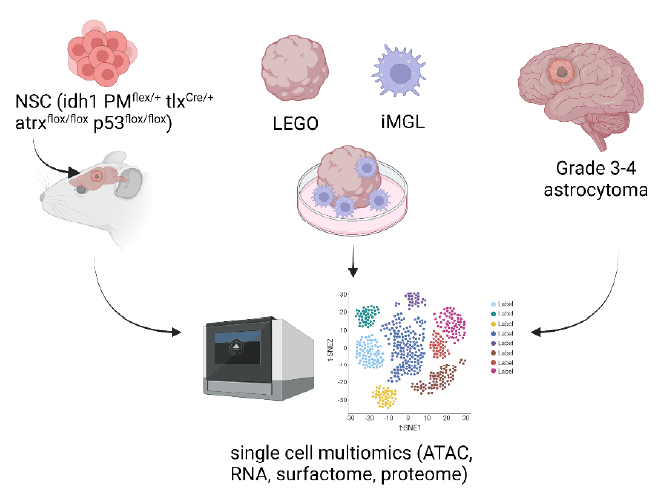
- Characterize TME following in vitro / in vivo induction of IDH and subsequent mutations in mutant IDH knock-in mouse models (IDH KI-M)
- Assess tumor genotype-specific microglia phenotypes using Laboratory Engineered Glioma Organoid (LEGO) – human microglia-like cell (iMGL) co-cultures
- Assess tumor genotype-specific surfactomes in astrocytomas, LEGO, IDH KI-M
Task 2
Assess and modulate intratumoral myeloid cell antigen presentation capacity in IDH-mutant gliomas
Steps / Workflow
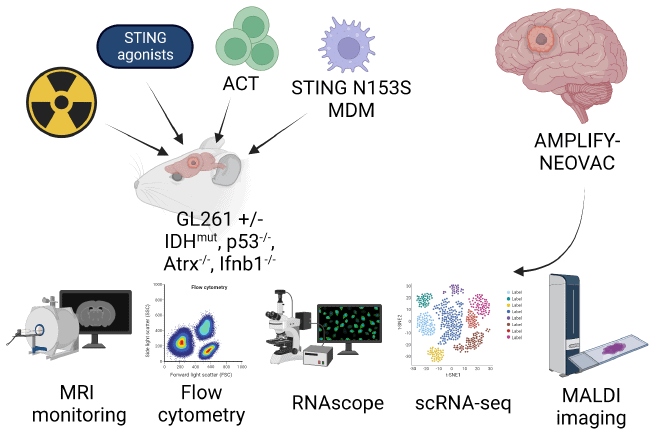
- Perform longitudinal profiling of CD45+ cells from experimental syngeneic glioma models following irradiation
- investigate response to STING agonists and MΦ transfer with constitutive active STING in combination with TCR-engineered T cells (ACT; OTI / OTII)
- comparatively assess APC of intratumoral myeloid cells and T cell landscape in AMPLIFY-NEOVAC
Task 3
Modulate semaphorine function in the context of T and myeloid cell interactions in IDH-mutant mouse model systems and in glioma organoids.
Steps / Workflow
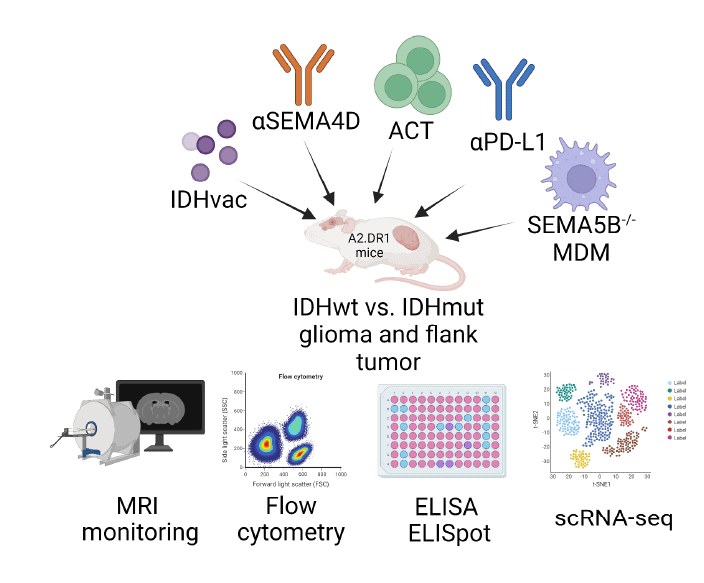
- Assess preclinical efficacy of combinatorial treatments in A2.DR1 flank tumors / gliomas and AMPLIFY glioma organoids by combinining
- Mutant IDH vaccination with SEMA4D antibody (MAb67 2)
- Mutant IDH – TCR – T cells with MAb67-2
- Anti-PD-L1 ICI with MAb67-2
- Assess immune modulatory capacities of icv . SEMA5B-KO MΦ
- Assess SEMA4D-blockade associated reprogramming of T and myeloid cell phenotypes in AMPLIFY-NEOVAC multicellular organoids

PUSCH, STEFAN, DR. RER. NAT.
University Hospital Heidelberg, Institute of Pathology, Dep. of Neuropathology, Im Neuenheimer Feld 224, 69120 Heidelberg, Germany

BUNSE, LUKAS, DR. MED.
University Hospital Mannheim, Department of Neurology, Theodor-Kutzer-Ufer 1-3, 68167, Mannheim, Germany
Address
Im Neuenheimer Feld 400
69120 Heidelberg
Themen
Research
- Focus A
- A01: Targeting tumor cell network communication to overcome primary and adaptive resistance in glioblastoma
- A02: Development of a specific combination therapy for histone H3-mutant pediatric glioblastoma
- A03: Deciphering resistance against targeted treatments
- A04: Elucidating tumor-associated microglia interactions in astrocytomas CNS WHO-grade 4
- A05: Predictive biomarkers for MGMT-promoter-methylated glioblastoma (2019 – 2023)
- A06: Resistance mechanisms of glioblastoma against alkylating agents and radiotherapy
- A07: Mapping and targeting neuron-tumor networks to tackle therapy resistance in glioblastoma
- A08: Personalized glioblastoma treatment guided by patient-derived tumor organoids
Research
- Focus B
- B01: Mechanisms of response and resistance to glioma-specific t cells
- B02: DNA mis-match repair regulates immune checkpoint blockade therapy in glioblastoma (2019 – 2023)
- B03: Targeting immunosuppressive programs in isocitrate dehydrogenase mutant gliomas
- B04: Impact of myeloid cells on the adaptive immune response in newly diagnosed and recurrent glioblastomas
- B05: Dissecting the response of glioblastoma and its tumor microenvironment to focused high-dose radiotherapy (2019 – 2023)
- B06: Visualization and characterization of immune responses in H3K27M mutant gliomas
Research
- Focus C
- C01: Comprehensive preclinical pharmacology testing of drugs used for glioblastoma treatment
- C02: Radiomics, radiogenomics and deep-learning in neurooncology
- C03: Imaging immune signatures of glioma response and resistance towards immunotherapy (2019 – 2023)
- C04: Metabolic signaling in glioblastoma: a spatial multi-omics approach
- C05: Overcoming glioma radio-resistance with particle therapy
- C06: Functional characterization of EGFR structural variants associated with long-term survival in glioblastoma, IDH-WT